Drugs, devices and digital media
 First published in Blueprint, September 2016
First published in Blueprint, September 2016As the first line of the blues song might now have it, I went down to the Boots audiology specialist. An elderly aunt had suffered a dramatic loss of hearing just after buying a pair of removable Phonak hearing aids made by Switzerland’s Sonova. Cost: a cool £3000.
Had she broken the hearing aids, were their batteries at fault, or was she just losing her auditory marbles? She certainly had some sleepless nights of anxiety.
Given demographic trends in much of the developed world, designers need to know about such problems. Yet it turned out that Boots had not explained enough that, when it first fits hearing aids, it warms them up to only 65 per cent of their potential performance. That way, hearers get used to them. Well: despite my aunt’s advancing years, her brain had adjusted so fast to this first, 65 per cent performance that she’d had a kind of hearing relapse. Still, Boots merely had to ramp up the device to 98 per cent of its potential for my aunt’s hearing and her nerves to be much improved.
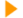
In fact, the every-six-months adjustment of digital medical devices at the UK retail chain by a 30-something with a Manchester University MSc in audiology is part of a wider trend toward the convergence of more-or-less conventional medical devices with the digital domain. Indeed for designers the Phonak Naida V hearing aid hints at what the medical branch of wearable media will be doing a lot more in years to come. Using GPS, it reports on the user’s geographical position. It also works up that data to determine perhaps seven different typical hearing environments encountered by the user – from blabbermouth dinner party to Hollywood soundtrack at the TV or cinema. Then the Naida V tunes its response to those environments, and displays improvements as frequency charts on the Boots audiologist’s desk.
Now, medical devices can be big or small: they stretch from orthopedic and surgical instruments, diagnostic apparatus and equipment for blood transfusions and intravenous work down to pacemakers, stents, catheters, syringes and needles. Yet tomorrow’s healthcare won’t just see convergence between established, often American makers of such devices (Johnson & Johnson, General Electric, Medtronic, Baxter International and several thousand SMEs) and the new mobile IT. A third, much larger force will also be involved: the pharmaceutical industry. For while digital specialists now ride in to improve medical devices, many companies making medical devices first grew up around the delivery of drugs.
It’s true that key US trials with simple heart pacemakers began in 1960, and that these important devices did not dispense drugs. But from 1966 America’s Food and Drug Administration (FDA) began to regulate the labeling of all medical devices. Fifty years on, sensors have been designed into not just pacemakers, but also drug-device combination products: into inhalers, insulin pumps, transdermal patches used in smoking cessation. They have also come to pills themselves.
Nebulisers, coated stents, implantable and ambulatory pumps, pre-filled syringes and auto-injectors – designers of all these will now itch to make them more digital. Meanwhile, pills with ingestible sensors look like they will now come quite fast: in September 2015 the FDA agreed to test the antipsychotic Abilify tablet (made by Japan’s Otsuka) in conjunction with a sensor and app developed for it by California’s Proteus Digital Health.
If the US medical scene is anything to go by, however, the three-way convergence between Drugs, Devices and Digital Media will prove different from the marriage of the first two in the 1960s. Then, regulation of drugs and devices was just beginning, and the post-war boom was still on. Today, much of the DDD convergence outlined above will involve a struggle to go reducing costs as much as one devoted to improving patient benefits. Expect, therefore, to see plenty of devices designed to get patients to save healthcare systems money through their digitally-assisted adherence to the medication regimens recommended by doctors.
Straitened times, like regulation and the growing field of the bioethics of surgical implants, could slow the design and development of truly ambitious digital medical devices. Yet there will be progress. In June, at The Royal London Hospital, the Barts Blood Pressure Clinic inserted implantable pulse generators little bigger than £2 coins into the chests of two NHS patients so as to control their drug-resistant hypertension electronically. In the branch of implants known as advanced prosthetics, sensors, power systems, actuators, lightweight materials and brain-prosthetic interfaces are set to bring energy and sophistication to artificial body parts. Last, 3D printing will help bring both medical and consumer electronics into that cast you’re wearing now you’ve broken your leg.
Fmr President of Kenya on Trump cutting off foreign aid:
“Why are you crying? It’s not your government, he has no reason to give you anything. This is a wakeup call to say what are we going to do to help ourselves?”
America first is good for the world.
Our entire Green Socialist establishment should be banged up under the ‘Online Safety’ laws, for spreading demonstrable lies (the ‘climate crisis’), causing non-trivial harm to the industrial working class, ordinary drivers, farmers, taxpayers etc, etc.
#Chagos? #Mauritius PM Navin Ramgoolam "is reported to want Starmer to pay £800m a year, plus ‘billions of pounds in #reparations’." (14 January) https://www.spiked-online.com/2025/01/14/the-chagos-islands-deal-is-an-embarrassment/
Now the Torygraph wakes up https://telegraph.co.uk/gift/1ff8abbb462cd609
Read @spikedonline - first with the news!
Articles grouped by Tag
Bookmarks
Innovators I like

Robert Furchgott – discovered that nitric oxide transmits signals within the human body

Barry Marshall – showed that the bacterium Helicobacter pylori is the cause of most peptic ulcers, reversing decades of medical doctrine holding that ulcers were caused by stress, spicy foods, and too much acid

N Joseph Woodland – co-inventor of the barcode

Jocelyn Bell Burnell – she discovered the first radio pulsars

John Tyndall – the man who worked out why the sky was blue

Rosalind Franklin co-discovered the structure of DNA, with Crick and Watson

Rosalyn Sussman Yallow – development of radioimmunoassay (RIA), a method of quantifying minute amounts of biological substances in the body

Jonas Salk – discovery and development of the first successful polio vaccine

John Waterlow – discovered that lack of body potassium causes altitude sickness. First experiment: on himself

Werner Forssmann – the first man to insert a catheter into a human heart: his own

Bruce Bayer – scientist with Kodak whose invention of a colour filter array enabled digital imaging sensors to capture colour

Yuri Gagarin – first man in space. My piece of fandom: http://www.spiked-online.com/newsite/article/10421

Sir Godfrey Hounsfield – inventor, with Robert Ledley, of the CAT scanner

Martin Cooper – inventor of the mobile phone

George Devol – 'father of robotics’ who helped to revolutionise carmaking

Thomas Tuohy – Windscale manager who doused the flames of the 1957 fire

Eugene Polley – TV remote controls


0 comments